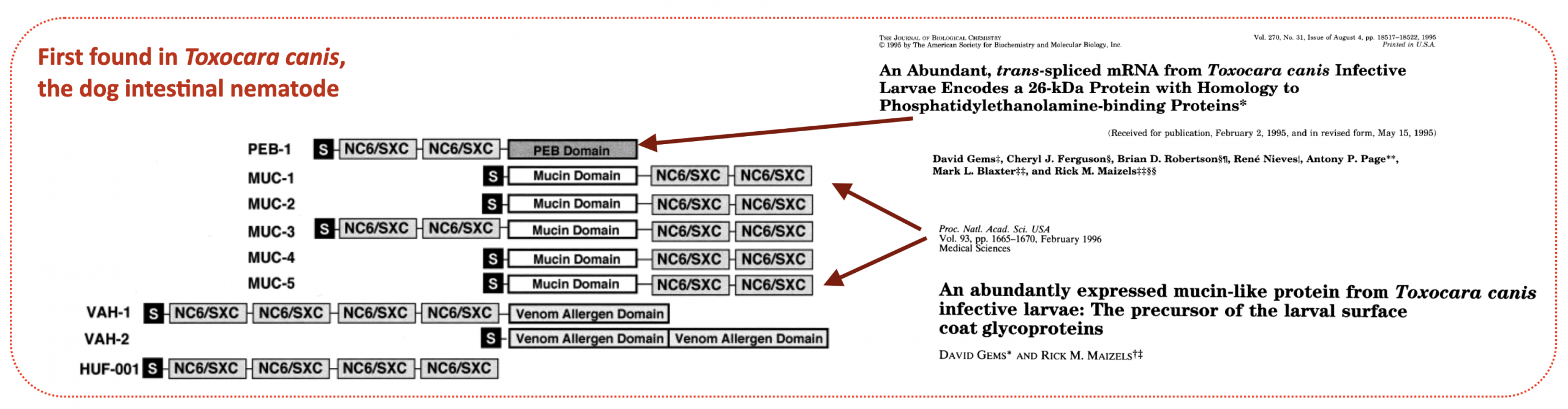
ShK proteins are named after a toxin from the sea anemone Stichodactyla helianthus that blocks a voltage-gated potassium channel (Kv). In helminths, this motif was originally discovered by David Gems in the intestinal nematode Toxocara canis (Gems et al., 1995; Gems et al. 1996) characterised as a 6-Cysteine module that was found in non-identical repeats. The original name “NC6” was for a short time replaced by “SXC”.and present in combination with other modules such as mucins, phosphatidylethanolamine-binding protein, and venom allergen homologues.
An ShK from the filarial nematode Brugia malayi has been trialled as a novel anti-inflammatory agent that blocks the Kv channel in T cells (Chhabra et al., 2014)
A prominent ShK from the enteropathogenic nematode Steinernema carpocapsae, is Sc-ShK-1 which when injected into Drosophila fruitflies, compromises their immunity to bacterial pathogens (Lima et al., 2022).